As lead vaccines announce good results and intentions to register for fast-tracked safety authorisation in EU and the US, immunologist Byram Bridle, reminds us of questions that they will need to answer:
Dr. Byram Bridle, PhD, Associate Professor of Viral Immunology, University of Guelph, Ontario, Canada
- How many of the total study subjects are being reported on? Partial results can range from being representative of the entire data set to being biased.
- How many study subjects had detectable immune responses and what was the magnitude?
- Were there antibody responses in the respiratory tract, which is where SARS-CoV-2 infects, and did these antibodies efficiently neutralize the virus?
- Were SARS-CoV-2-specific T cells induced? A balanced anti-viral response should include antibodies to prevent infection and T cells to kill viruses that get past the antibody barrier.
- Did the immune responses have a ‘Th1’ or ‘Th2’ bias? The former type of immune response is optimal against viruses, the latter is usually sub-optimal and sometimes even dangerous in the context of respiratory viral infections.
- Did the vaccine confer long-term immunological memory? A prophylactic vaccine may be useless without this. If immunological memory is short-lived, vaccinated individuals could become susceptible to infection before enough people are immunized to achieve ‘herd immunity’. Another term for this is ‘duration of immunity’ (i.e. how long does immunological protection last?)
- How did the vaccine perform in senescent animals and/or elderly humans? Those most in need of protection against COVID-19 are the elderly and immunocompromised.
- How was safety assessed and what were the results?
- Have the results been published for review by other scientists? If not, when? It is recommended to publish in open-access journals, which are available to the public. Comments merely reflect opinions unless there are validated data to back them up.
- Related to #6 above, what is the plan to manufacture and roll-out enough vaccine doses to achieve herd immunity in any given country? What is the realistic timeline for this? If >1 year, there is no way to know if COVID-19 vaccines will confer protection for this long because they didn’t exist one year ago. The development of every historical vaccine took >4 years, so there were years-worth of ‘duration of immunity’ data. For example, optimistic projections suggest ~1 billion doses might be possible by the end of 2021, but for two-dose regimens, that means only 500 million people could potentially be vaccinated in just over one year. With the global population at 7.8 billion people, that represents only 6% of the world’s population. The lowest estimate to achieve herd immunity is 60%.
- How will equitable distribution of vaccines be accomplished? For example, pre-order waiting lists seem to be dominated by developed countries; are any developing countries on these waiting lists?
- What is the cost of a full vaccine regimen going to be? Is it affordable for developing countries? Some epidemiologists have predicted that efficient roll-out of vaccines to developing countries will require the price to be <$6 US.
- Storage conditions for vaccines could impact distribution and market competitiveness. What data are available to support the claimed storage conditions? For example, the default storage temperature for RNA in research laboratories is -80o This is based on a plethora of scientific evidence that RNA is more stable at this temperature compared to -20oC.


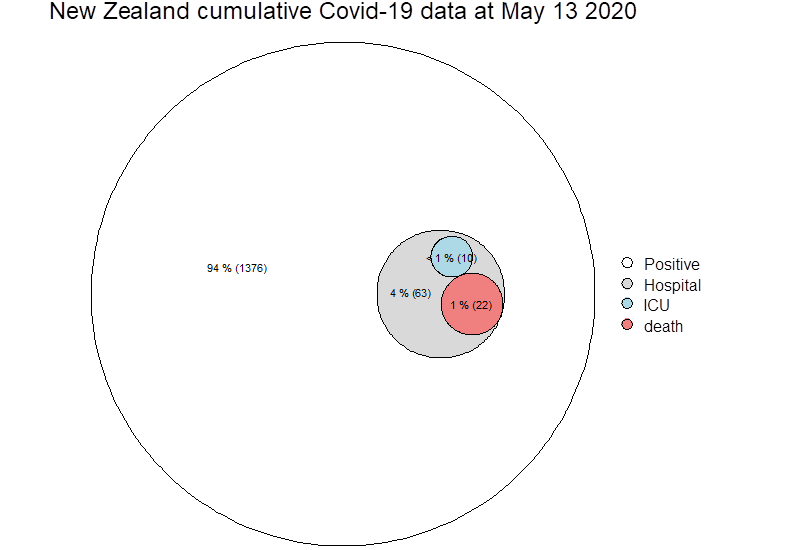
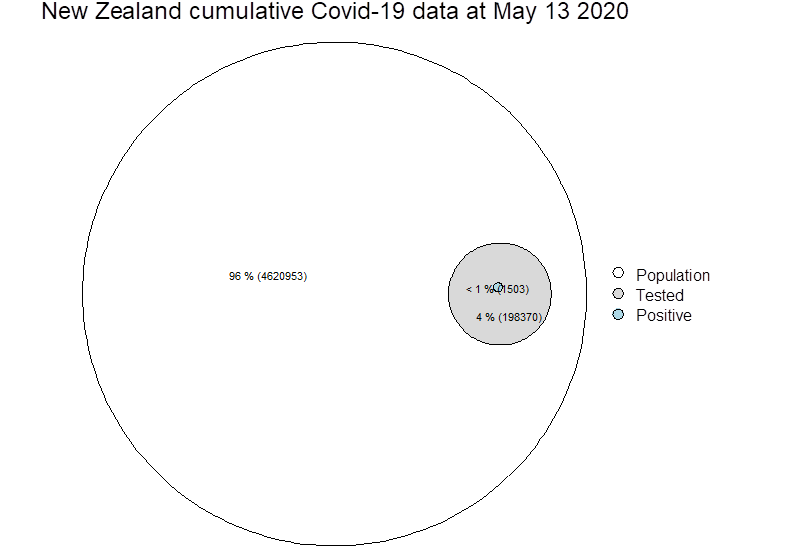 Zeroing on test positive cases (blue circle above, now below), it is not possible from paper to know how many deaths actually went to ICU, so these cells may not be mutually exclusive…
Zeroing on test positive cases (blue circle above, now below), it is not possible from paper to know how many deaths actually went to ICU, so these cells may not be mutually exclusive…